| Fig5102 |
 |
| Fig5103 |
Fig5101
Active mutation of Ras, an oncogene product, is found in nearly 30% of all human cancer cases. Extensive studies have revealed that Ras functions as a molecular switch of many important signaling cascades, such as cell growth, differentiation, and apoptosis. Sustained signaling from mutated Ras is one of the chief causes of cancer. Ras is a very simple switch (Fig. 5101). When it is bound to GDP, it is inactive. When it is bound to GTP, the conformation change in the effector region induces binding to and activation of many target molecules, including serine/threonine kinase Raf. The activation, i.e., replacement of GDP with GTP, is mediated by many guanine nucleotide exchange factors (GEFs), which are controlled by phosphorylation, calcium, lipids, and so on. The activated Ras is then inactivated by its intrinsic GTPase activity; however, this activity usually requires an additional protein, GTPase activating protein (GAP).
| Fig5102 |
 |
| Fig5103 |
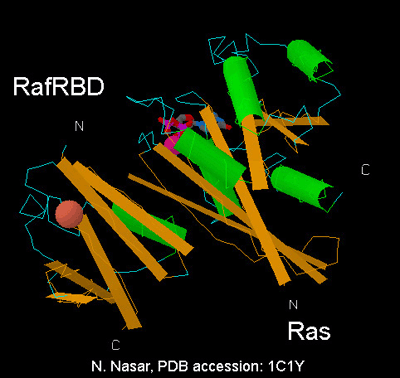
Raichu uses Ras as the sensor region and Raf as the ligand region (Fig. 5102). Upon GTP binding, the effector region of Ras binds to Raf, which increases FRET from CFP to YFP (Fig. 5102). The structure of the Ras-Raf complex has been resolved (Fig. 5103). As is clear from these data, the C-terminus of Raf and the N-terminus of Ras are placed in close vicinity to each other. So, in the initial attempt, we placed Raf at the N-terminus of Ras. A partial list of trials is shown in Fig. 5.1.5. Unfortunately, all these trials failed: GTP-loading did not affect FRET efficiency. So, we placed Ras at the N-terminus of Raf, which finally gave us the prototype Raichu. An important lesson from this experience is that the structure will help us to design the probe, but should not be relied upon too much. The nucleotide sequences of Raichu-Ras and Raichu-Rap1 have been deposited in DDBJ (AB046925, AB051846).
Plasmid Information:
pRaichu-101 (H-Ras, no CAAX box), sequence
pRaichu-102 (H-Ras, Y40C mutant, no CAAX box), sequence
pRaichu-103 (H-Ras, G12V mutant, no CAAX box), sequence
pRaichu-104 (H-Ras, S17N mutant, no CAAX box), sequence
For Raichu-Ras, only one amino acid at the C-terminus of YFP was removed, whereas in Raichu-Rap1, the removal of the 11amino acids at the C-terminus of YFP gave us the best result. The longer the spacer between Rap1 and Raf, the higher the increase in FRET efficiency upon stimulation in our hands.
Raf is not an optimal target molecule of Rap1 in a physiologic milieu. When we used RalGDS, which has high affinity to Rap1, for the ligand region, high FRET efficiency was observed even without any stimuli. Consequently, external stimuli can scarcely increase FRET efficiency. Since most protein-protein interaction is mediated by a combination of constitutive and regulated binding, we can hardly predict which ligand will work best as a partner in PHOGEMON. Similarly, there is no rule for the size of ligand region. Usually, finding the optimal ligand region is the hardest part of producing PHOGEMON.
We have introduced many mutations in Ras. By killing the GTPase activity, we can make a constitutively active Raichu-Ras, which of course has high YFP/CFP ratio. Other interesting mutants include ts Ras. With this mutation, Raichu-Ras is stable only at low temperatures.
We check the following.
Sensitivity to GEF and GAP. According to our test results, the sensitivity to GEFs and GAPs was exactly the same between Raichu-Ras and the authentic Ras.
Correlation of GTP (%) on Raichu-Ras with the YFP/CFP ratio. From 5% to 80% of GTP-Raichu-Ras, the FRET efficiency (YFP/CFP), and GTP-loading are in a linear correlation.
Correlation of GTP (%) on Raichu-Ras with that of endogenous Ras. The GTP-loading of Raichu-Ras is always higher than that of the authentic Ras. This is probably because Raf competitively inhibits GAP. However, the GTP-loading of Raichu-Ras and the endogenous Ras show a linear correlation.
Effect on the authentic signaling cascades. As far as we tested, Raichu-Ras does not affect the endogenous signaling cascades in a short-term observation. However, we failed to establish cell lines that express high level of Raichu-Ras, suggesting some toxicity to the cells.
 |
| Fig5104 |
 |
| Fig5105 |
Understand the principles of fluorescence microscopy and FRET. The instruments are far from cheap. Buy only what you really need.
Start with the easiest experiment. We recommend the use of Raichu-Ras, COS1, and EGF stimulation for beginners. You can easily find out whether or not EGF truly stimulated the cells by looking for membrane ruffles. Avoid confluent cells.
Continue maintaining the easiest system. When you have trouble with your own cells and your probe, you will want to have a positive control.
Train your eyes. For many cell types, fluorescence does not provide enough light for the naked eyes. If you waste too much time searching for a good cell, the probe will be photobleached. You must find cells that are appropriate for the observation as soon as possible.
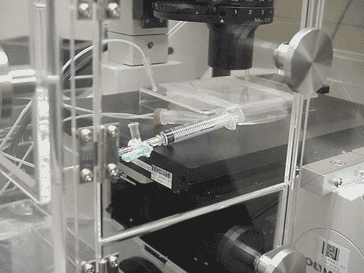
Set up a chamber system. You need to warm up the chamber system for more than 1 hr before the experiment (Fig. 5104). Injecting growth factors often makes the cells appear out of focus. If you do not have a autofocusing system, you may need to set up a good injection system (Fig, 5105).