 |
| Fig5402 |
 |
| Fig5403 |
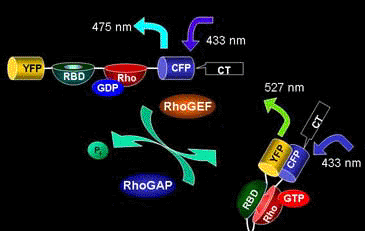
Rho-family G proteins are regulated by the three classes of protein; guanine nucleotide exchange factor (GEF), GTPase activating protein (GAP), and guanine nucleotide dissociation inhibitor (GDI). GEF promotes the exchange of GDP with GTP, which results in the binding of the G proteins to their effector proteins. Typical GEF proteins in this family consist of a Dbl-homology (DH) domain, which exhibits GEF activity, and additional domains that mediate interaction with peptides or lipids. The GTP on activated Rho-family G proteins is hydrolyzed in the presence of GAP to resume the GDP-bound inactive state. GDI not only competes with GEF but also holds Rho-family G proteins in the cytoplasm. Therefore, the dissociation of GDI is a prerequisite for the membrane association and activation of Rho-family G proteins. ERM-family proteins, which are involved in cell-to-cell interaction, release Rho from Rho-GDI, translocating Rho from the cytoplasm to the membrane.
 |
| Fig5402 |
 |
| Fig5403 |
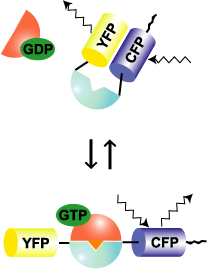
Raichu-RhoA uses human RhoA as the sensor regions and Rho-binding domain (RBD) of PKN as the ligand region (Fig. 5402). Upon GTP binding, the effector region of RhoA binds to PKN, which increases FRET from CFP to YFP. In contrast to Raichu-Ras and Raichu-Rap1, PKN was placed before RhoA. We constructed probes with the RBDs of Rhotekin, mDia, and Rhophilin and with both orders. The selected construct yielded the best gain. The comparison of such trial probes has been described [3]. The probe is anchored to the membrane via the C-terminal region of RhoA itself or that of K-Ras, which is fused to CFP. Since Raichu-RhoA cannot bind to RhoGDI, these probes cannot monitor the effect of RhoGDI. It should be remembered that the probe monitor the balance between GEFs and GAPs at the membrane. To overcome the insensitivity to RhoGDI, Raichu-RBD was developed (Fig5403). The original idea of this probe was proposed by Chalk et al [1]. Probably due to the nature of GFP to form homo-dimer, FRET can be detected by simply sandwiching RBD with CFP and YFP. Fortunately for us, binding of the endogenous RhoA to RBD was found to reduce the FRET efficiency. Thus, this probe can monitor the effect of RhoGDI as well as GEF and GAP. It should be pointed out, however, that the gain of this type of probe is significantly lower than that of Raichuu-RhoA probe.
Fusion to the C-terminal region of RhoA is preferable in terms of its delivery to the places where the endogenous RhoA (unbound to RhoGDI) should be. As reported by many researchers, the C-terminal region of RhoA (or CAAX box) delivers proteins preferentially to the intracellular membrane compartments, whereas K-Ras CAAX delivers proteins preferentially to the plasma membrane. To the best of our knowledge, many stimuli increase RhoA activity at the plasma membrane, but not at the endomembrane compartements. If you do not think the endomembrane compartment is of particular interest, placing the probe at the plasma membrane is preferable, because it will reduce the background signal from the endomembrane compartments.
MOV files run on Quick Time Player. MPEG files run on Windows Media Player.
Activity change of RhoA MOV 7.8 Mb MPEG 1 Mb
Activity change of Rac1MOV 3.5 MbMPEG 1 Mb
Activity change of Cdc42 MOV 4.5 Mb MPEG 1 Mb
1. Graham DL, Lowe PN, and Chalk PA. A method to measure the interaction of Rac/Cdc42 with their binding partners using fluorescence resonance energy transfer between mutants of green fluorescent protein. Anal Biochem 296:208-217, 2001.
2. Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol 162:223-232, 2003