1. What is Crk?Å@
| |
| Fig5201 |
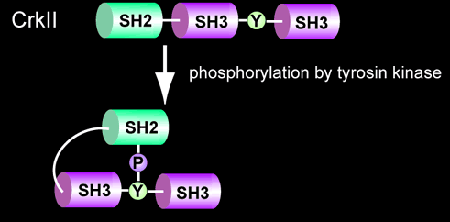
In 1988, Bruce J. Mayer of Hidesaburo Hanafusa's laboratory at the Rockefeller University discovered the v-crk oncogene from CT10 retrovirus [1], which was isolated in 1937 by a Nobel laureate, Albert Claude [2]. The Crk oncogene product consisted only of the Src homology (SH) 2 and SH3 domains (Fig. 5201). Crk revealed two important secrets about signal transduction. One, the Src homology regions, which were known as regulatory domains conserved among tyrosine kinases, are actually components shared by many signaling molecules. Second, the SH2 domain functions to regulate protein-protein interaction in a manner dependent on tyrosine phosphorylation [3]. Following this success, many domains, such as PTB, WW, have been identified and shown to mediate protein-protein interaction as the SH2 domain. The mammalian homolog of v-Crk, CrkII, is phosphorylated on Tyr221 upon stimulation. The SH2 domain binds intramolecularly to this tyrosine 221. It is believed that this intramolecular binding of SH2 to Tyr221 regulates CrkII signaling negatively [4].
2. Structure of Picchu
| |
| Fig5202 |
| |
| Fig5203 |
| |
| Fig5204 |
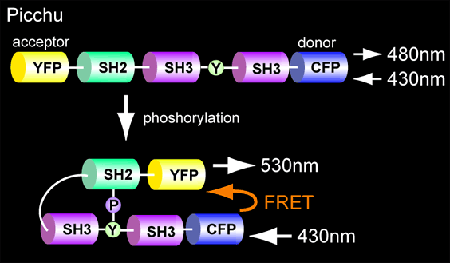
Picchu differs from Raichu in that both the sensor and ligand regions are derived from CrkII (Fig. 5202). Phosphorylation of tyrosine 221 induces intramolecular binding of the SH2 domain of CrkII, which will increase FRET from CFP to YFP. We deleted the C-terminal SH3 domain to obtain optimal FRET (Fig. 5203).
Picchu-911 (wild type) sequence, MAP
Picchu-911X (CAAX) sequence, MAP
3. Ligand region
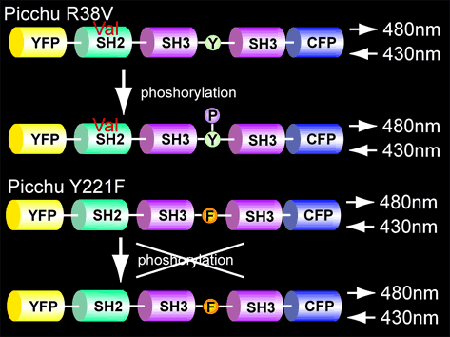
To verify that FRET was caused by intramolecular binding of the SH2 domain of CrkII to phosphorylated Tyr221, we replaced Arg38, which is critical for the recognition of phosphotyrosine with Val. As expected, in this mutant, phosphorylation of Tyr221 did not change the FRET efficiency (Fig. 5204). This mutant is versatile in confirming that the increase in FRET reflects CrkII phosphorylation during acquisition of the live images.
4. Sensor region
Similarly, Tyr221Phe mutant can be used as a control (Fig. 5204).
5. SH3 mutant:
In Picchu, the N-terminal SH3 is intact so that Picchu expression may have interfered with the authentic signal transduction cascades. Thus, in an improved Picchu, the landmark Trp of SH3 is replaced with Leu.
6. Membrane-localized Picchu
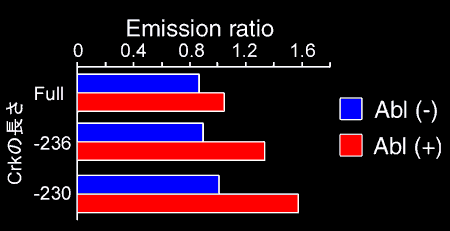
What we have learned from Picchu is that the probe moves rapidly in the cells. We usually take 2 images per minute. One minute is enough for a soluble protein to move from one edge to the other. Even if phosphorylation of CrkII occurs in a very restricted area, we can observe diffuse increases in the phosphorylated CrkII. To overcome this problem, we anchored Picchu to the membrane by fusing the CAAX box of Ki-Ras protein at the C-terminus (Fig. 5205). Using this probe, Picchu-X, we were able to increase the sensitivity and time resolution. The increase in space resolution enabled us to observe that CrkII phosphorylation starts from the cell periphery.
7. Confirmation of the versatility of Picchu
We have checked that the phosphorylation of Picchu and the authentic CrkII correlates nicely.
8. References
- Mayer BJ, Hamaguchi M, Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature 1988; 332:272-5.
- Claude A. Preparation of an active agent from inactive tumor extracts. Science 1937; 85:294-5.
- Matsuda M, Mayer BJ, Fukui Y, Hanafusa H. Binding of oncoprotein, P47gag-crk, to a broad range of phosphotyrosine-containing proteins. Science 1990; 248:1537-9.
- Feller SM, Knudsen B, Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J 1994; 13:2341-51.